Clean in process, is an integral part of all industries where food & liquor is prepared for human consumption. Pharmaceutical & medicine industries also carry out CIP. In general depending upon industry, CIP is done 1-3 times in a day. Whenever process stops or processing ingredient is changed CIP is required. Standard cycle of CIP has acid & base wash, followed by rinse & disinfection using chemical or steam. Finally post chemical dis-infection rinse of 1-2 cycles is necessary.
There are three main areas which gets contaminated & infected & needs serious disinfection.
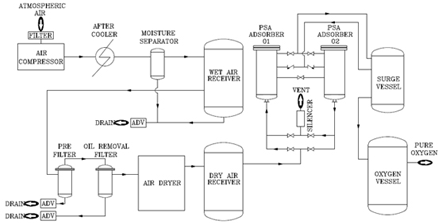
Using control dose of air Ozone keeps air disinfected. Ozone being strongest air disinfectant works very quickly & does not produce any harmful by-products. Also unused Ozone gets self destructed into harmless Oxygen & the process can be expertise by Ozone destruct techniques.
Highly sensitive
Modality sensitive
Pharmaceutical & clean room
Poultry
Beef pork mutton
Dairy, chocolate, ice cream
Bakery & confectionery
Packed food & tin food
Sea food
Wine beverages
Electronic
Aerospace
Nuclear research